Double mutant DNMT3A AML: a unique subtype experiencing increased DNA damage and poor prognosis
Mutation of DNMT3A, encoding a de novo methyltransferase essential for cytosine methylation, is a common early event in clonal hematopoiesis (CH) and adult acute myeloid leukemia (AML). Spontaneous deamination of methylated cytosines damages DNA, which is repaired by the base excision repair (BER) enzymes MBD4 and TDG. Congenital MBD4-deficiency has been linked to early-onset CH and AML, and is marked by excessive levels of DNA damage and mutation of DNMT3A. Strikingly, wildtype (WT) DNMT3A binds TDG, thereby potentiating its repair activity. Since TDG is the only remaining BER enzyme in MBD4-deficient AML patients, we investigated whether mutant DNMT3A negatively affects the capacity of TDG to repair methylation-linked DNA damage.
MBD4 and TDG repair activity was assessed in vitro in the presence of varying concentrations of recombinant wildtype or mutant DNMT3A (R635W, R688C, R882C, A884V) using glycosylase assays. Differences in survival and co-mutation was assessed in our AML cohort comprising 1.872 DNMT3A WT, 585 SM and 88 DM patients. Whole-genome sequencing (WGS) was performed on bone marrow aspirates from 22 AML patients, comprising 6 DNMT3A WT, 7 SM and 9 DM patients to assess whether methylation-linked DNA damage increases dependent on the DNMT3A mutation type or number. Alphafold 2.0 was used to model the interaction between DNMT3A and TDG to determine the impact of DNMT3A mutations positioned at the interaction interface.
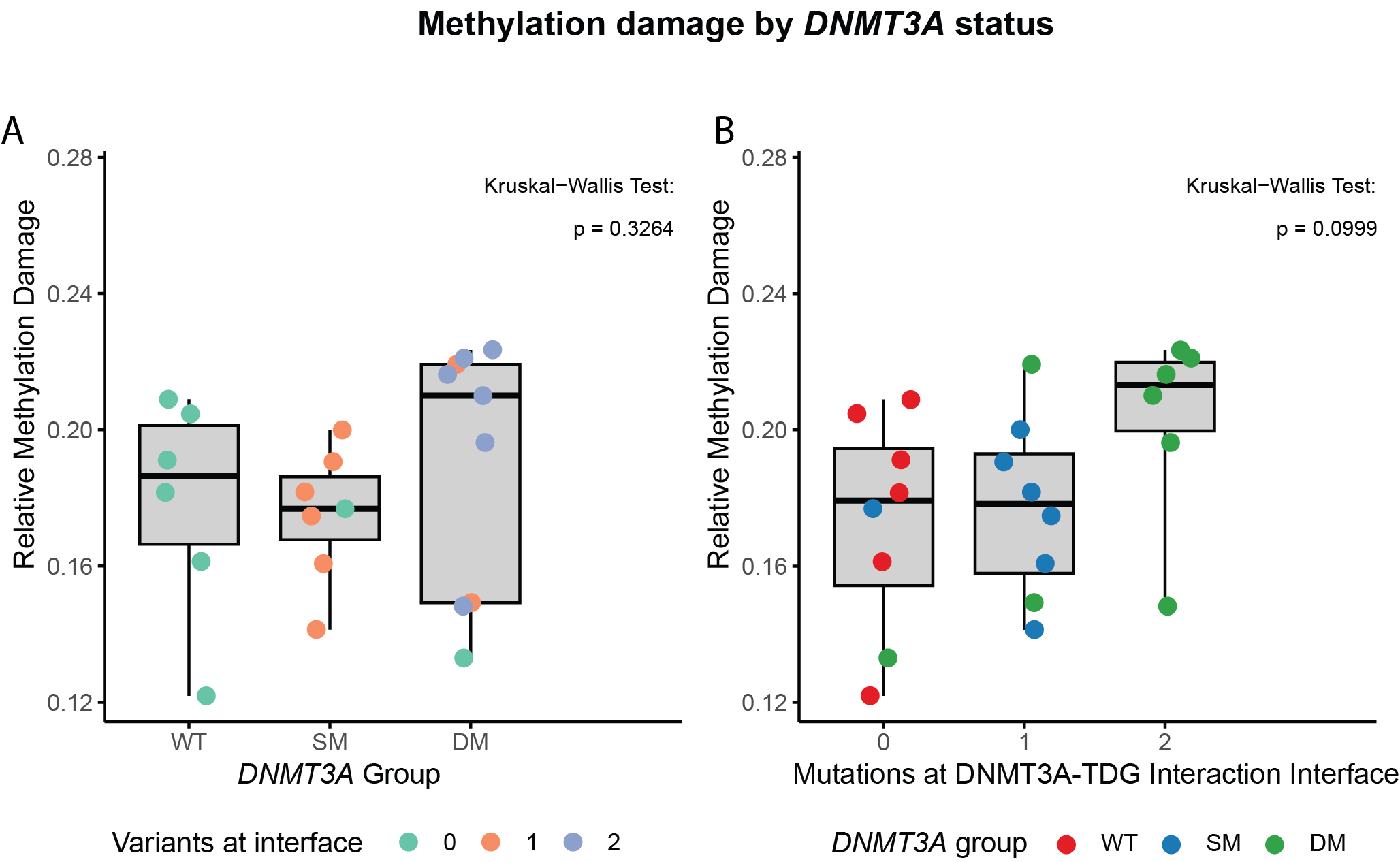
We found that, whereas WT DNMT3A stimulates TDG function, mutant DNMT3A impairs TDG-mediated repair of DNA damage in vitro. In light of this finding and to extrapolate our observations to the broader AML patient population, we investigated the genetic profiles and survival outcomes of AML patients with single (SM) versus double mutant (DM) DNMT3A. DM DNMT3A AML patients show a characteristic driver mutation landscape and reduced overall survival when compared to SM DNMT3A AML patients. Importantly, whole-genome sequencing showed a trend for increased DNA damage in primary DM DNMT3A AML samples, especially when DNMT3A mutations are located at the DNMT3A-TDG interaction interface.
We show that biallelic mutant DNMT3A, a subgroup comprising approximately 4% of the complete AML population, is linked to increased methylation-linked DNA damage, diverging driver landscape and unfavorable overall survival.