Dynamics of Circulating Tumor DNA in Relapsed/Refractory DLBCL Patients
Relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) often has poor outcomes, with 74% of patients not responding to salvage chemotherapy and a median survival of 4.6 months. New biomarkers are needed to identify patients unlikely to benefit from salvage therapy and to tailor treatment strategies. Circulating tumor DNA (ctDNA) has shown potential as a prognostic marker in DLBCL, but data on its role in R/R cases during salvage chemotherapy are limited. This study aimed to evaluate ctDNA dynamics in R/R DLBCL using low-coverage whole-genome sequencing, a rapid, cost-effective approach.
Twenty five patients with R/R DLBCL diagnosed between 2019 and 2022 in the University Medical Center Groningen were included. Plasma samples were collected at the time of R/R disease diagnosis (n = 25) and during second-line chemotherapy (n = 86). Clinical data were retrieved from patients' files. Low-coverage whole-genome sequencing of cell-free DNA was conducted on all plasma samples; QDNAclinic was to identify copy number variants (CNVs)and IchorCNA to determine the estimated tumor fraction (ETF).
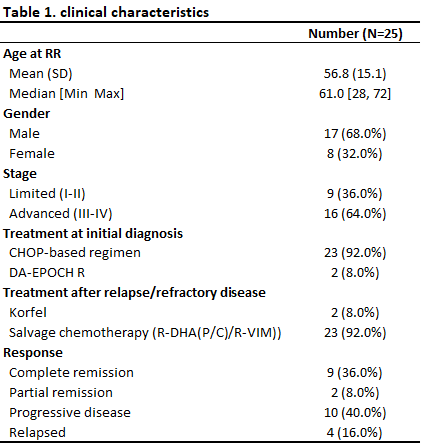
The median age of R/R DLBCL patients was 61 years (range: 28–72); 17 patients were male (68%), and 16 (64%) presented with Ann Arbor stage III-IV disease. Second-line chemotherapy included R-DHAP for 23 patients, while 2 cases with CNS involvement received high-dose methotrexate-based chemo. The outcomes of the second-line treatment were: complete remission (CR) in 9 patients (36%), partial remission (PR) in 2 patients (8%), progressive disease (PD) in 10 patients (40%), and relapse after CR in 4 patients (16%) (Table 1).
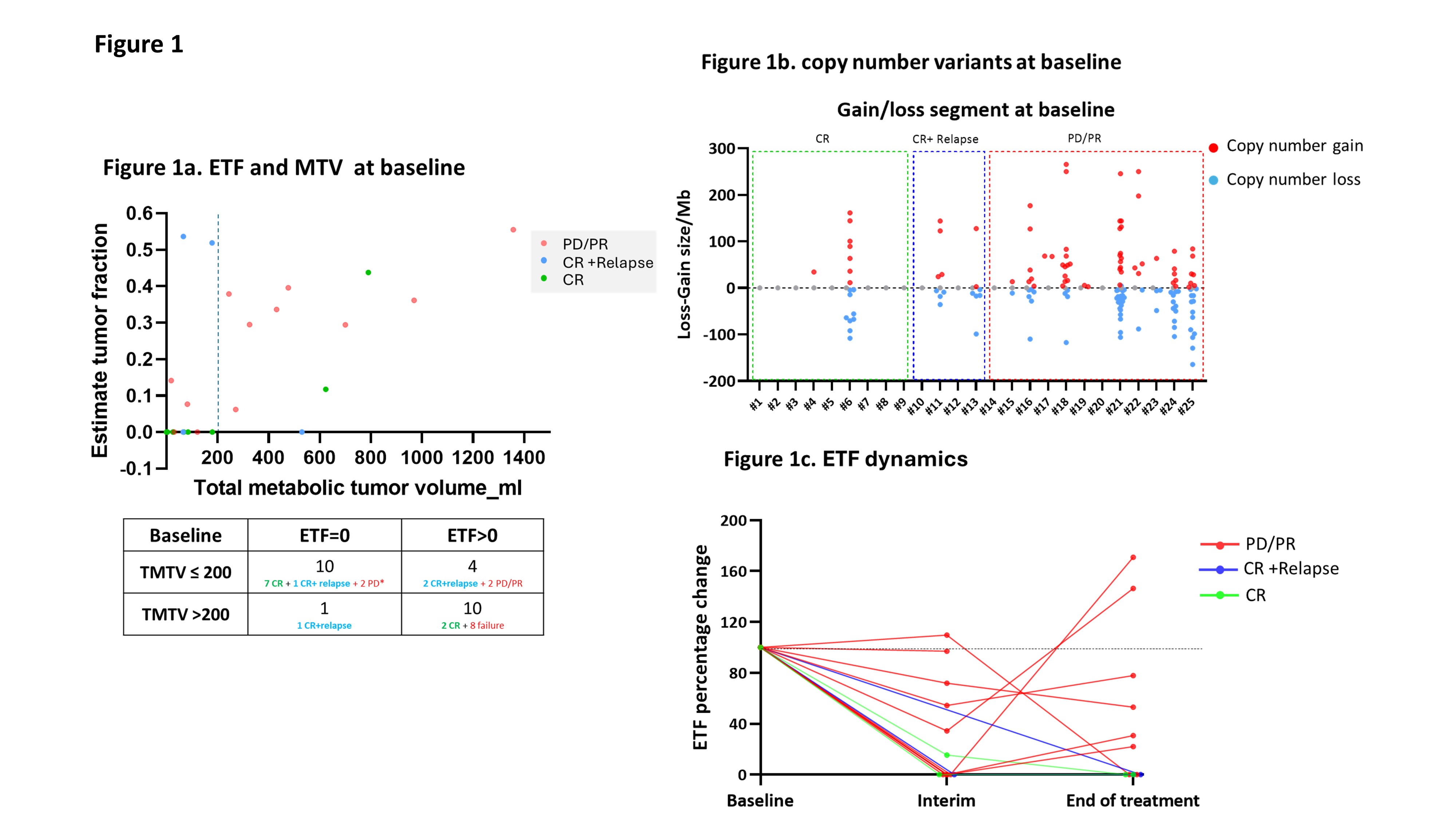
At the time of R/R disease, ctDNA was detected in 14 of the 25 cases (56%). A modest correlation was observed between ETF and TMTV (r² = 0.35). Detectable ctDNA (ETF > 0) was associated with poor outcomes in second-line therapy. Specifically, 8 of 10 patients (80%) with both ETF > 0 and TMTV > 200ml experienced PD/PR. In contrast, 7 of 10 patients (70%) with no ETF and low TMTV achieved long-term remission (Figure 1a). Furthermore, patients with a higher number of CNVs at baseline had worse treatment outcomes compared to those with fewer CNVs (Figure 1b).
For patients with detectable ctDNA at baseline, ctDNA dynamics were monitored at baseline, interim, and the end of treatment. Inferior prognosis was associated with the presence of detectable ctDNA during or at the end of treatment, whereas patients who achieved ctDNA clearance demonstrated more favorable outcomes (Figure 1c).
A low number of CNVs or undetectable ctDNA at baseline are associated with favorable outcomes in R/R DLBCL. Detectable ctDNA during or after treatment correlates with poorer responses to second-line chemotherapy, highlighting ctDNA’s potential as a biomarker to guide treatment decisions in R/R DLBCL.