Cost-Effectiveness of Frontline TKI Strategies for Chronic Myeloid Leukemia Patients: in Pursuit of Treatment-Free Remission and Dose Reduction
Chronic myeloid leukemia (CML) management now includes dose-reduction (DR) and treatment-free remission (TFR). Evaluating cost-effectiveness of lifelong-prescribed expensive tyrosine kinase inhibitors (TKIs) for CML is crucial. Prior cost-effectiveness evaluations state that imatinib is the favorable frontline TKI. Some of these evaluations address TFR, but not DR, nor ageing and second-generation (2G)TKIs upcoming patent-expirations. This study evaluates cost-effectiveness of frontline TKIs for CML patients including these factors.
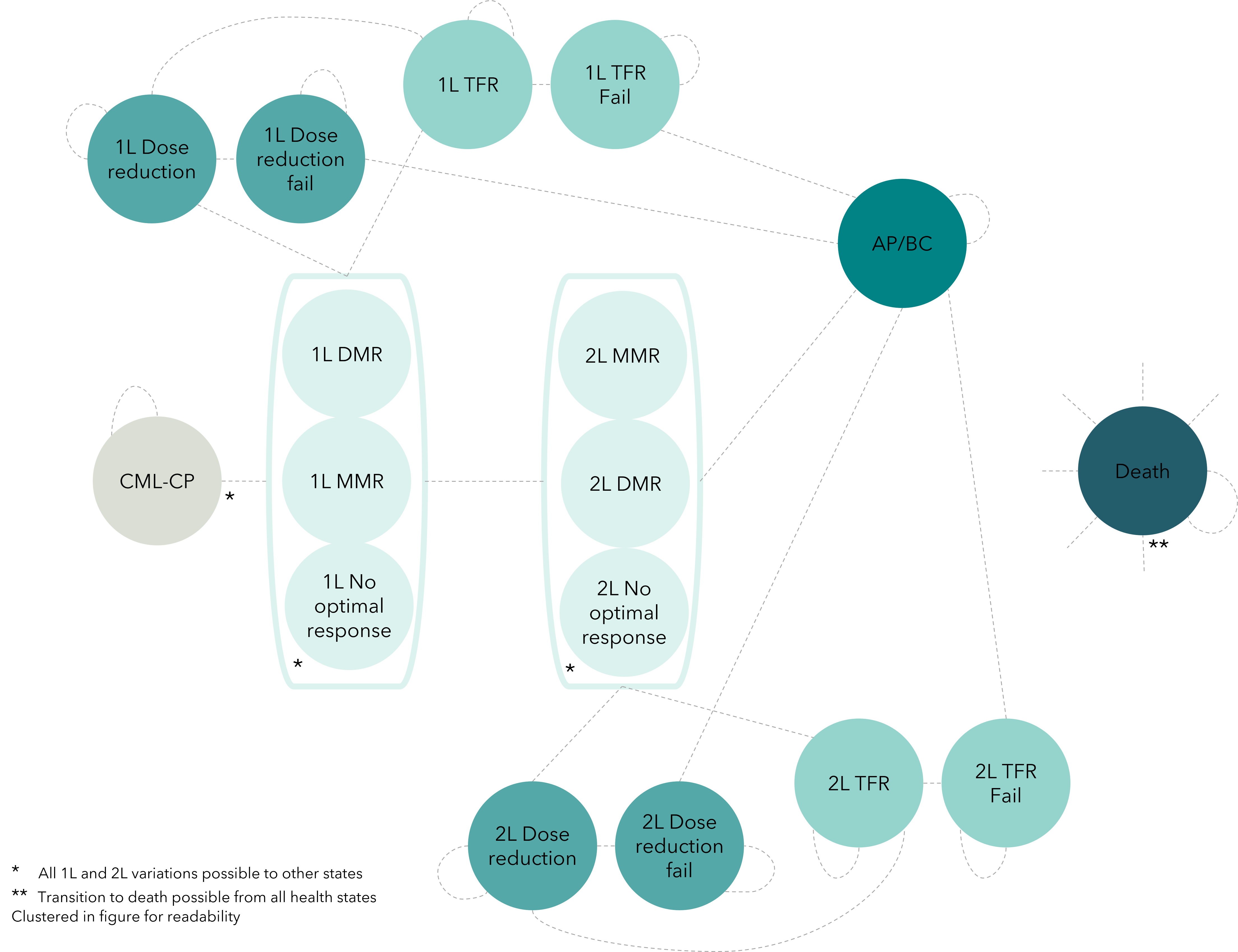
This Markov model evaluates cost-effectiveness of frontline TKIs for newly diagnosed CML patients using 17 health states. Transition probabilities, costs and utilities were derived from literature data. Incremental cost-effectiveness ratios were calculated. Sensitivity analysis and model validation were conducted.
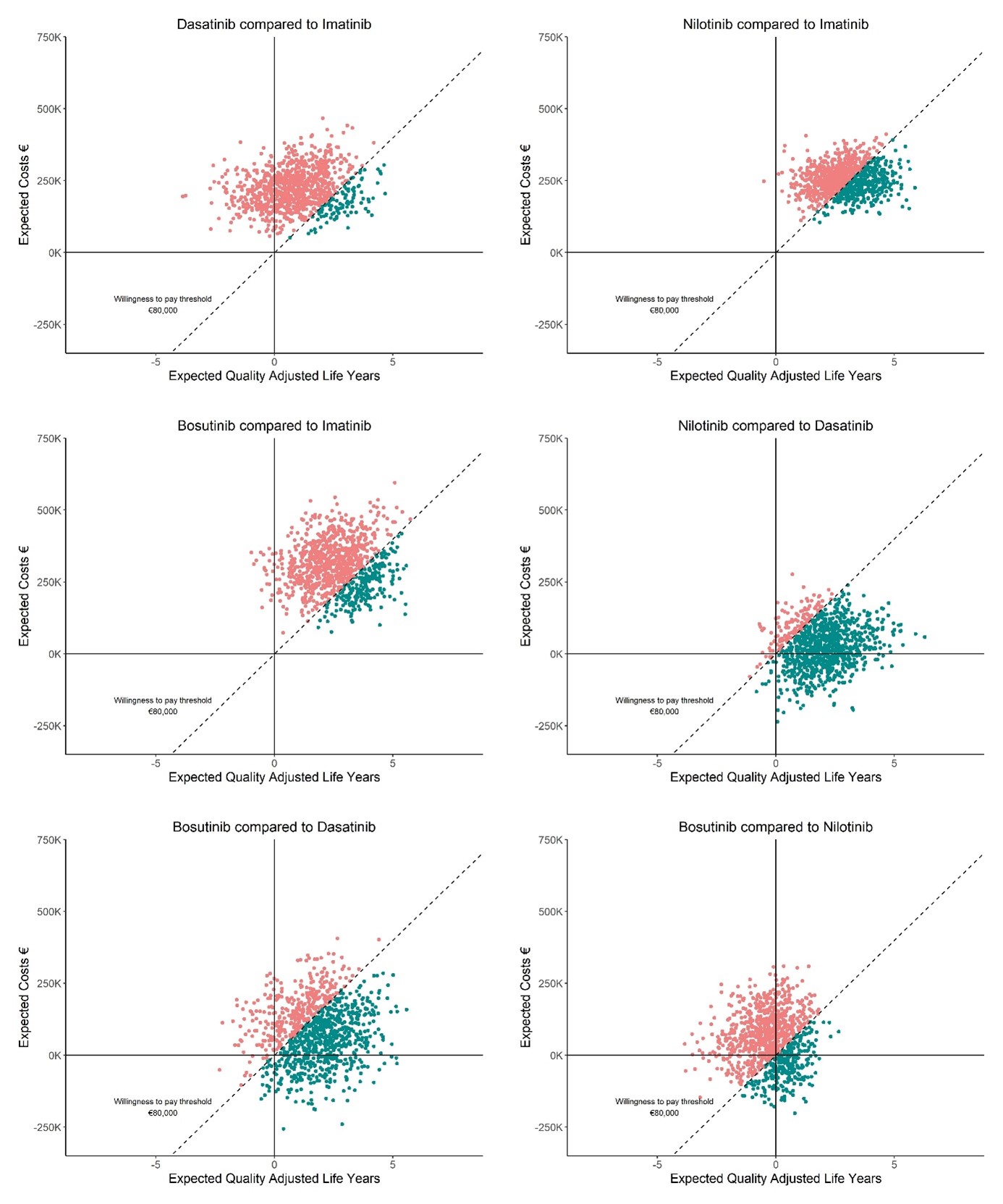
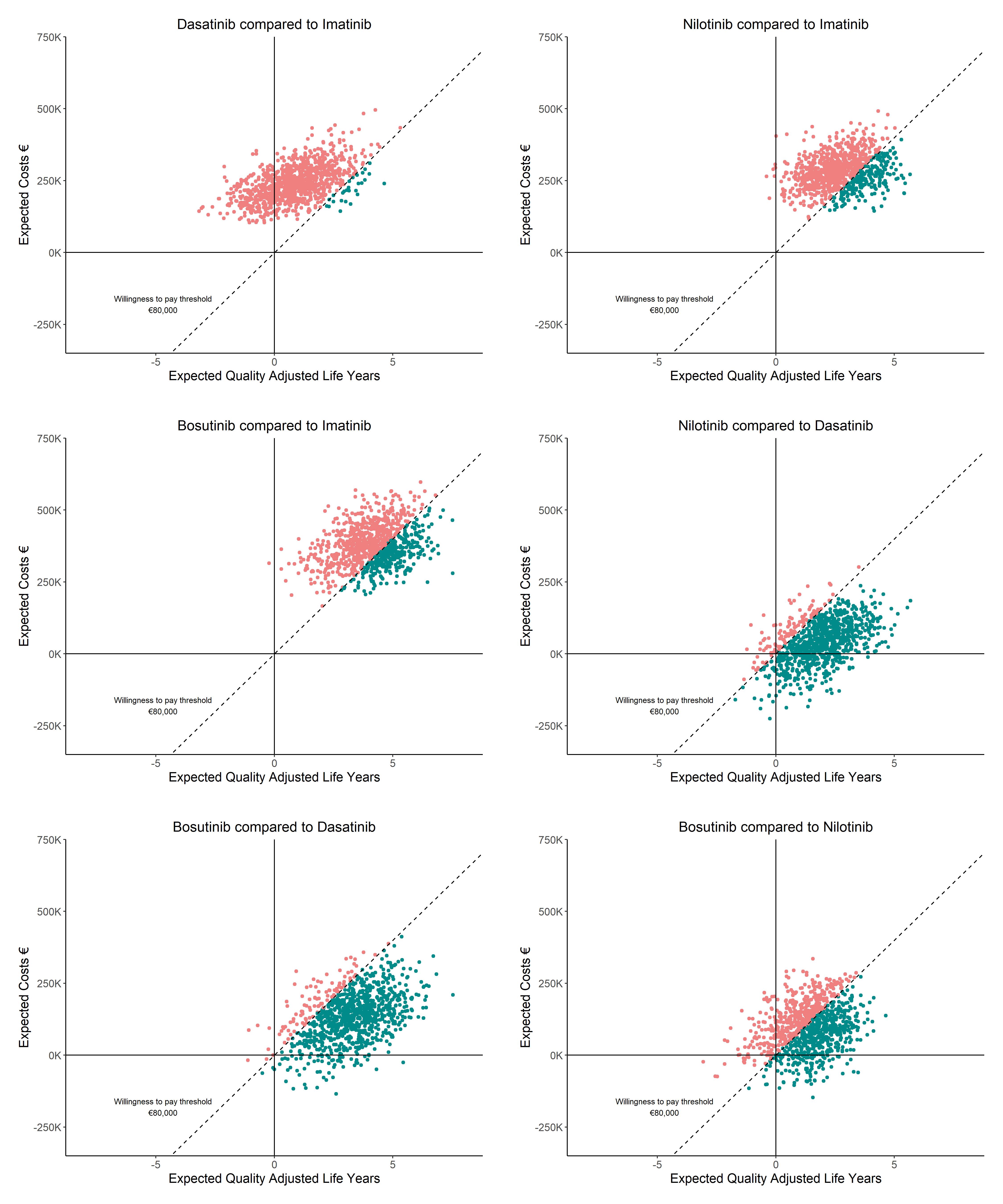
Nilotinib is most effective (20.13 QALYs) and imatinib is least effective (17.25 QALYs) for the model including TFR and DR. Imatinib was favored over dasatinib (89.80%), nilotinib (62.70%), and bosutinib (78.40%), at a WTP of €80,000/QALY. Without TFR and DR, fewer QALYs were generated. For patients at age 70, imatinib has a high probability of being cost-effective compared to dasatinib, nilotinib and bosutinib. With 50% 2GTKI cost reductions, nilotinib is considered cost-effective compared to imatinib (98.40%), dasatinib (94.80%) and bosutinib (68.90%).
Findings indicate that 2GTKIs excel in yielding QALYs, also for older (age>70) patients. Given current TKI prices, imatinib remains cost-effective. Including DR and TFR in CML-management generates more QALYs. Cost-reductions from expected 2GTKIs patent-expirations will greatly increase their cost-effectiveness. Results may inform 2GTKIs cost discussions after patent-expiration, potentially broadening global availability. Findings also emphasize the importance of aiming for TFR and DR in CML-management.