Monitoring multiple myeloma treatments in the Netherlands
Treatment effectiveness for real-world multiple myeloma (MM) patients vary greatly and multiple new medicines have been introduced over the last years, changing the treatment combinations and sequences of MM over time. Due to the introduction of these newly developed therapies, 5-year overall survival has doubled over the past decade to around 54%. However these new therapies come with high costs and at time of introduction the effectiveness is determined in a restricted population included in clinical trails only. Therefore, the NVvH and NVZA initiated the Dutch MM Treatment monitor in 2021 in order to gain insight in the effectiveness of MM treatments in clinical practice. The objective of our study was to assess the feasibility of construction a national monitor based on a real-world multiple myeloma population, focusing on use of real-world treatments.
An automated monitoring system is executed by PharmIntel and supervised by a board of hospital pharmacists and haematologists. From pseudonymised administrative healthcare data the following treatment related parameters are collected: medicine dispensing data and indication; patient parameters: age, gender, DOT and date of death. These data were used to calculate for each treatment: number of patients, treatment line, median duration, time-to-next-used treatment. Using digital algorithms, patient journeys and treatment sequences were constructed and were presented via a dynamic dashboard which allows correction for patient variables. Moreover, it is possible to benchmark outcomes between one's own hospital and other groups of hospitals.
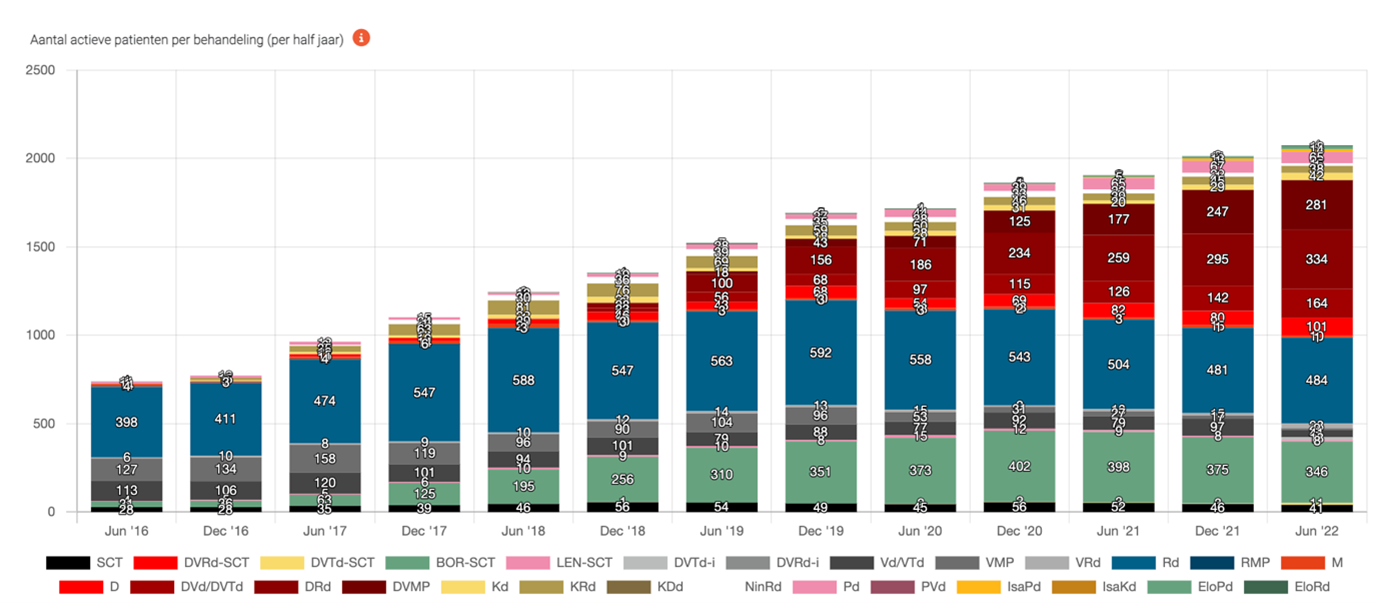
In the period from 2012 – 2022, 50 Dutch hospitals participated in the MM monitor and uploaded data from in total 8477 patients. A cohort is created in which a total of 5761 patients started their first therapy after 2012, 2798 patients received a 2nd line and 1320 and 997 patients were treated with a 3rd and 4th line and further lines, respectively. Treatments changed significantly over time as shown in figure 1 and since the dashboard is dynamic, many analyses are possible such as median treatment duration per line or time to next used treatment.
the Dutch MM Treatment Monitor is successfully constructed using real world data obtained from administrative healthcare data sources. Since the majority of the Dutch multiple myeloma patients is represented in this monitor, these data can be used to obtain insight of national use of newly introduced therapies in clinical Dutch practice.