Venous and arterial thrombosis incidence in the multiple myeloma HOVON-50, HOVON-87 and HOVON-95 trials
Multiple myeloma (MM) is associated with an increased venous thromboembolism (VTE) risk. A recent meta-analysis observed a 6.2% risk in lenalidomide-based regimens despite thromboprophylaxis. In the general population the incidence rate (IR) of first VTE in people aged ≥ 20 is 1.43 per 1000 person‐years. Current guidelines recommend aspirin or low molecular weight heparin (LMWH) thromboprophylaxis for low- and high-risk patients, respectively. MM may be associated with an increased arterial thrombosis (AT) risk. In the Netherlands in 2013 the ischemic stroke (IS) and myocardial infarction (MI) incidence for people aged 65-80 years was 28.4 and 49.8 per 10.000 inhabitants (cbs.nl), respectively.
A pooled analysis of the trials HOVON-50, HOVON-87 and HOVON-95 was performed. HOVON-50 was a randomized phase III study on the effect of Thalidomide combined with Adriamycin, Dexamethasone and High Dose Melphalan (HDM). HOVON-87 was a randomized phase III trial in elderly patients with previously untreated symptomatic MM comparing Melphalan/Prednisone (MP)-Thalidomide followed by thalidomide maintenance versus MP-Lenalidomide followed by lenalidomide maintenance. HOVON-95 was a randomized phase III study to compare Bortezomib-MP with HDM followed by Bortezomib, Lenalidomide, Dexamethasone consolidation and lenalidomide maintenance.
Follow-up started at induction and lasted 1 year. The pooled cumulative VTE, IS and MI incidences with death as competing risk were calculated. The IS and MI IR were calculated by dividing total events by total patient-years.
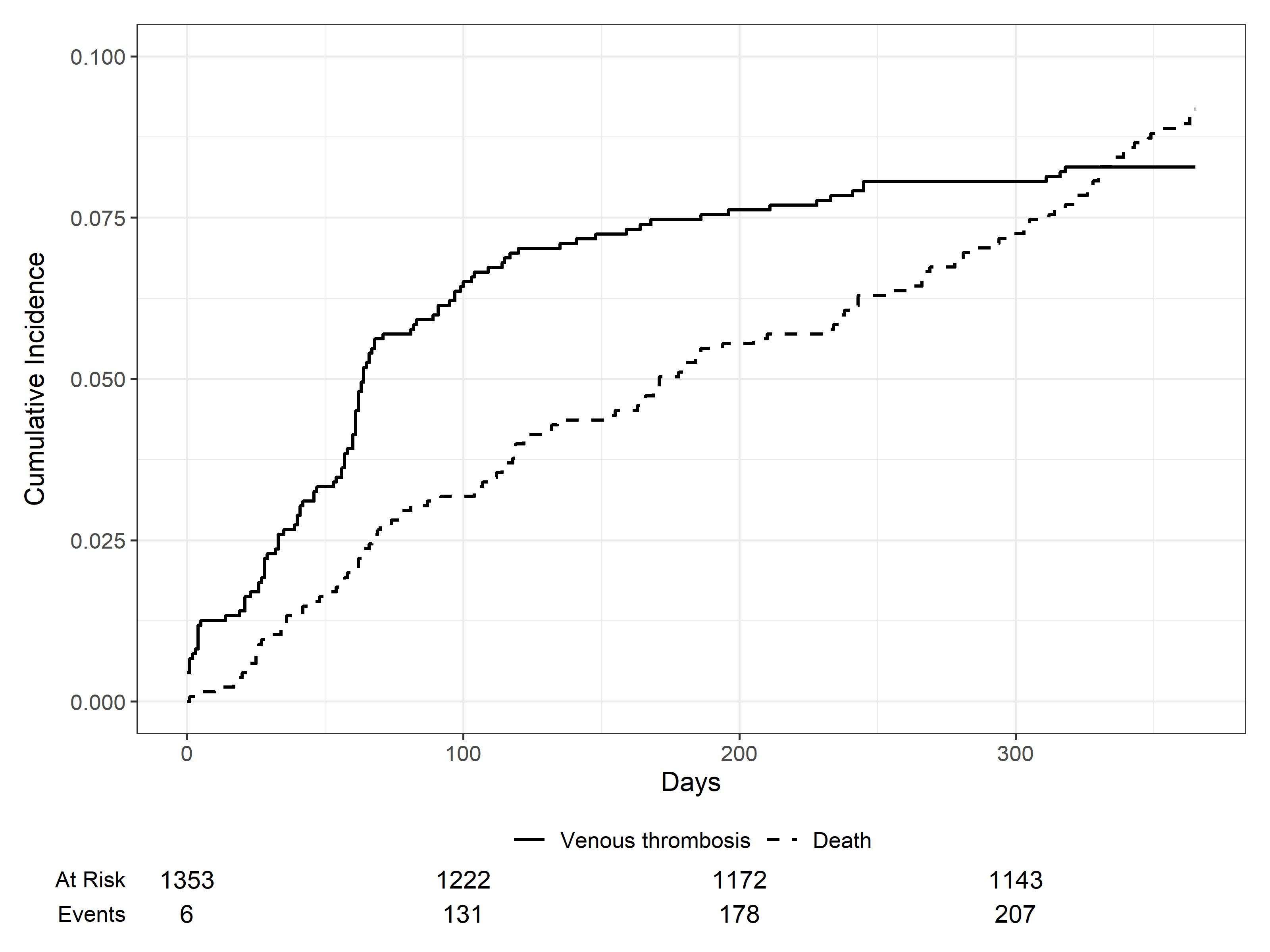
1353 Dutch MM patients were included in the final analysis. Median age was 61 (IQR 16), 59% were men and 55% received immunomodulatory-drug (IMID)-based induction. 60% of patients received antithrombotics during induction (33% aspirin, 24% LMWH and 3% vitamin K antagonist (VKA)). 112 patients experienced a VTE. The pooled 1-year VTE incidence was 8.1 (95% CI, 6.7, 9.6) (figure 1). The VTE proportion among patients with and without antithrombotics was 7.5% and 9.4%, respectively.
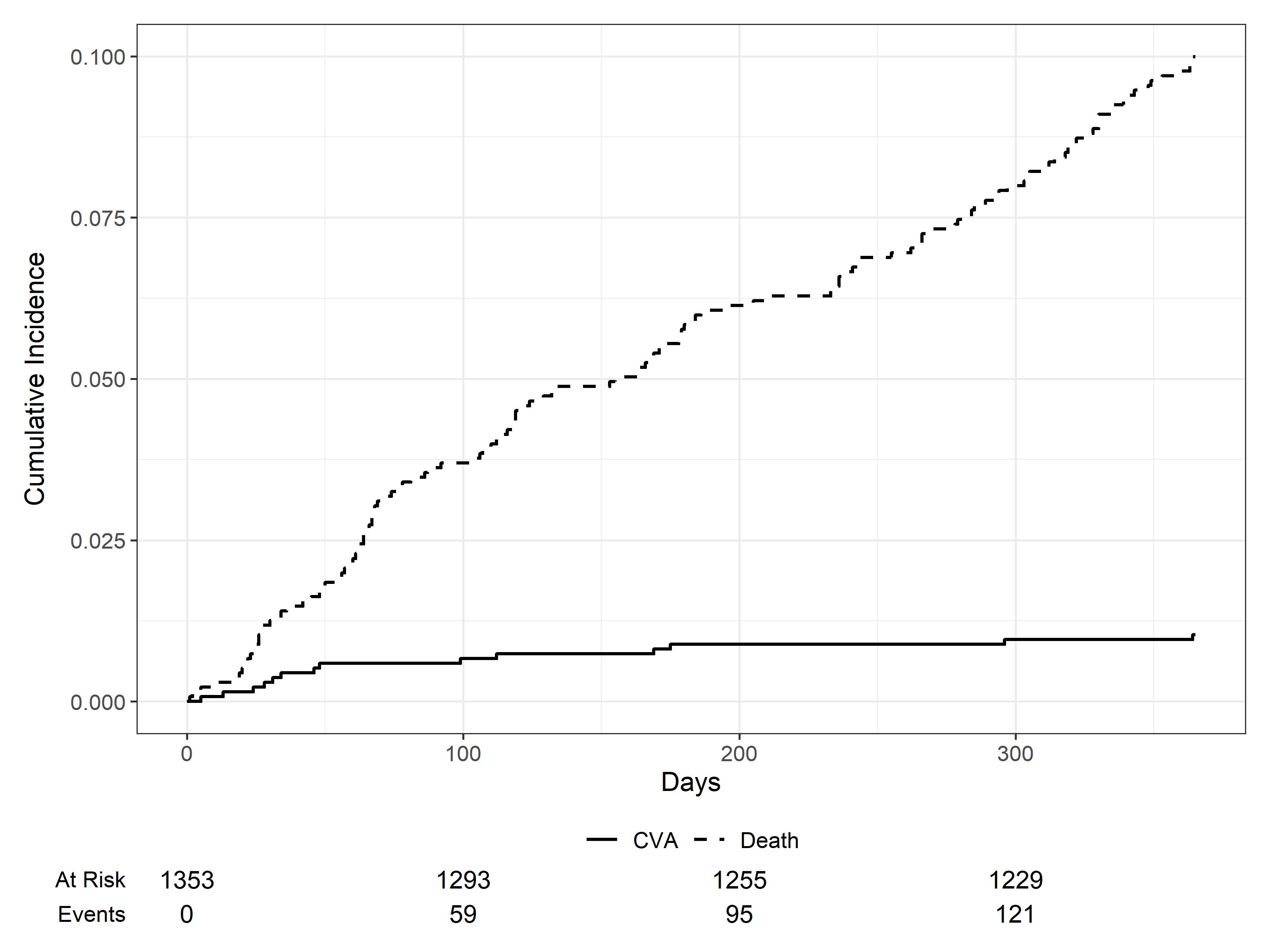
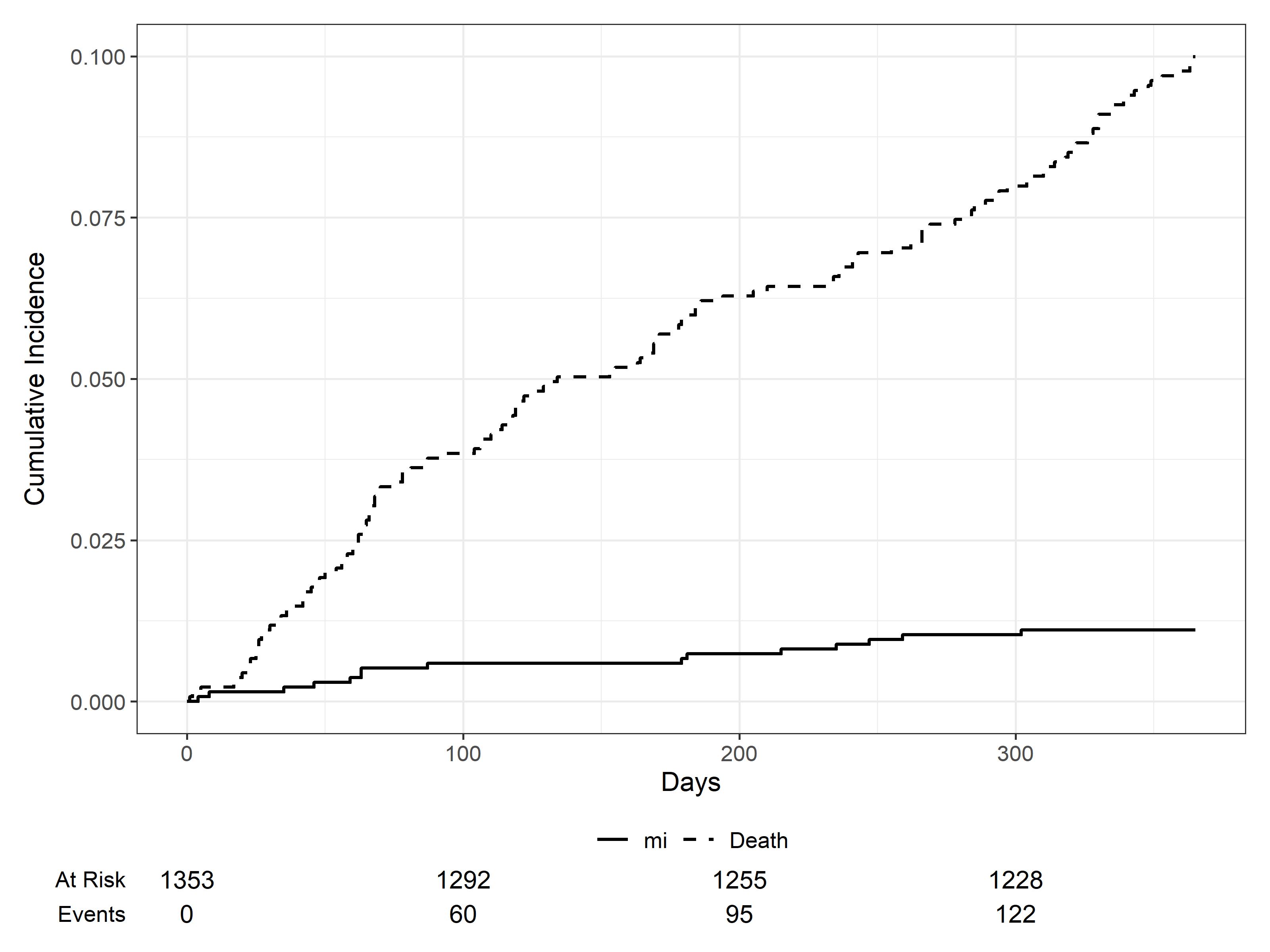
13 patients experienced an IS. The pooled 1-year IS incidence was 0.9 (95% CI, 0.5; 1.5) (figure 2) and the IR was 102 per 10.000 patient-years. 12 patients experienced a MI. The pooled 1-year MI incidence was 0.8 (95% CI, 0.4, 1.4) (figure 3) and the IR was 95 per 10.000 patient-years. One patient experienced both an IS and MI. A higher proportion of patients that experienced any AT were men (70% vs 59%), were over 65 (50% vs 34%), had diabetes (17% vs 8%) and received IMID-induction (75% vs 55%). The AT proportion among patients with and without antithrombotics was 2.7% and 0.7%, respectively.
Despite antithrombotic therapy, the VTE incidence remained high. MM patients may have a higher IS and MI risk than the general population. More attention should be given to whether patients with cardiovascular risk factors require additional prophylaxis.