Adaptive Designs in Randomized Clinical Trials: Reanalysis of the HOVON-87/NMSG18 Multiple Myeloma Trial
Randomized controlled trials (RCTs) are the gold standard to assess clinical efficacy of a novel drug, but extended follow-up time due to improved survival challenges their feasibility. We hypothesized that the efficiency of RCT conduct may be enhanced by adaptive trial designs, offering early anticipation of outcomes, potentially accelerating decision-making. We retrospectively evaluated how information from adaptive designs including interim analyses, could have facilitated early detection of efficacy or futility within the phase III randomized HOVON-87/NMSG18 trial, which compared melphalan-prednisone with either thalidomide (MPT-T) or lenalidomide (MPR-R) in 637 patients with newly diagnosed multiple myeloma (MM). The final analysis showed no statistically significant difference in progression-free survival between the treatment arms (Hazard Ratio [HR]: 0.87; 95%CI: 0.72–1.04; p=0.12).[Zweegman, et al., 2016]
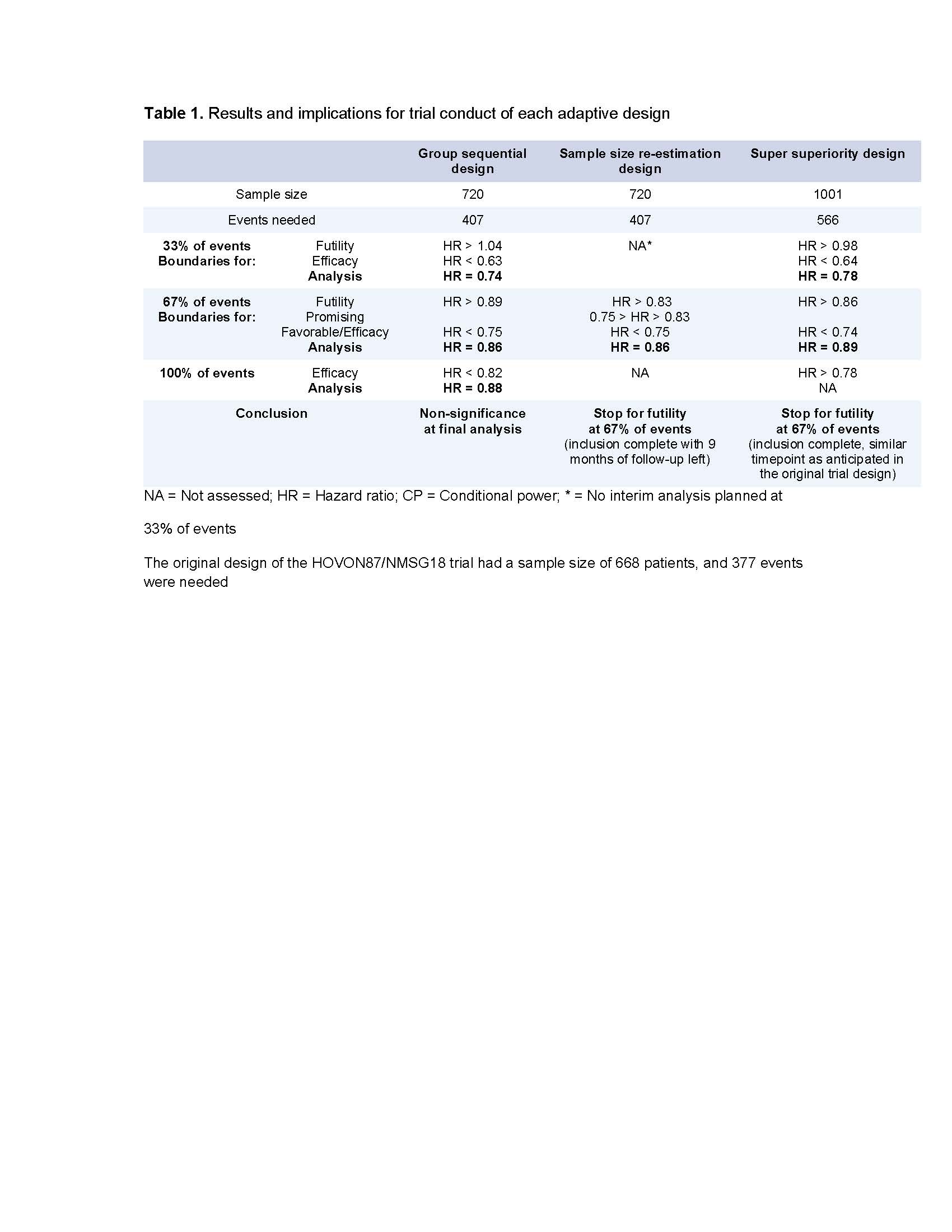
In this reanalysis, we used the HOVON-87/NMSG18 trial as a model to implement three adaptive trial designs: (1) group sequential, (2) sample size re-estimation and (3) super superiority designs, which recommend trial termination in case of strong efficacy or futility but continue when such signals are not evident. Efficacy and futility were assessed at 33% and 67% of events required for the final analysis, with a gamma spending function to control for false positive/negative results. Sample size re-estimation design was evaluated after 67% of events and includes the possibility to increase the sample size in case of promising but inconclusive results. The super superiority design tests against a null hypothesis of HR=0.95 instead of HR=1.0, aiming to detect a more profound clinical benefit.
Group sequential design: HRs at interim analyses were 0.74 (33% of events) and 0.86 (67% of events), both within the futility and efficacy boundaries, indicating continuation of the trial as planned.
Sample size re-estimation design: At 67% of events, the HR of 0.86 exceeded the predefined threshold for futility (0.83). Although inclusion was complete after 67% (n=273) of events, stopping the trial would have reduced follow-up time by 9 months compared with the original trial assumptions.
Super superiority design: A larger sample size (n = 1001) was required, due to the lower null hypothesis. At 67% (n=379) of events, the HR of 0.89 exceeded the futility boundary, which was at a similar time-point as anticipated in the original trial design.
Reanalysis of a MM trial suggests that adaptive designs can facilitate earlier decision making with predefined efficacy and futility thresholds. Such early signals could have reduced the follow-up time or offer the potential to increase the sample size if the effect is promising but not yet conclusive. We offer an overview for enhancing the efficiency of future clinical trials by enabling earlier detection of lack of efficacy, ultimately leading to more timely decisions.